

These changes can take place upon adding some external energies, temperature, and pressure. A phase is a distinctive form of a substance, and matter can change among the phases.As we know, that matter exists in four states: Solid, liquid, gas, and plasma, but on earth, matter exists in only three states.The matter is anything that has mass and occupies space.Another illustration is the change on a chilly, windy winter day from ice to water vapor instantly. Sublimation: Sublimation is the process through which dry ice transforms from solid carbon dioxide to carbon dioxide gas. Silver vapor deposition onto glass to create a silver mirror is another illustration. Fog in the mirror: When the temperature gets cooler and less than the spheric temperature, the gas or the vapors are changed to fog.Ĭondensation: Dew is a common result of condensation caused by airborne water vapor.ĭeposition: Hoarfrost is a grayish-white frost that develops in cold, clear conditions when water vapor freezes.

How did the droplets come on it? The reason is that the particles inside the substances are condensed.Ĭondensation is a phenomenon in which the complete heat is removed from the substance, and from there, it converts to liquid. Whenever we are tired off, or we come home after playing in the ground, we feel thirsty, and we come over to the fridge and open the door. Whenever the water droplets in the cloud mix, the cloud becomes heavy to pour down the droplets. Condensation:Ĭondensation is the transformation from which water vapor converts to liquid. This happens when the gaseous substance is cooled. For example: when water vapor (gas) present in the atmosphere changes to a liquid. The process in which the physical state of matter changes from the gaseous state to the liquid state is known as condensation. In reverse, when gas changes to a solid without going into the liquid phase, the process is called deposition or re-sublimation.
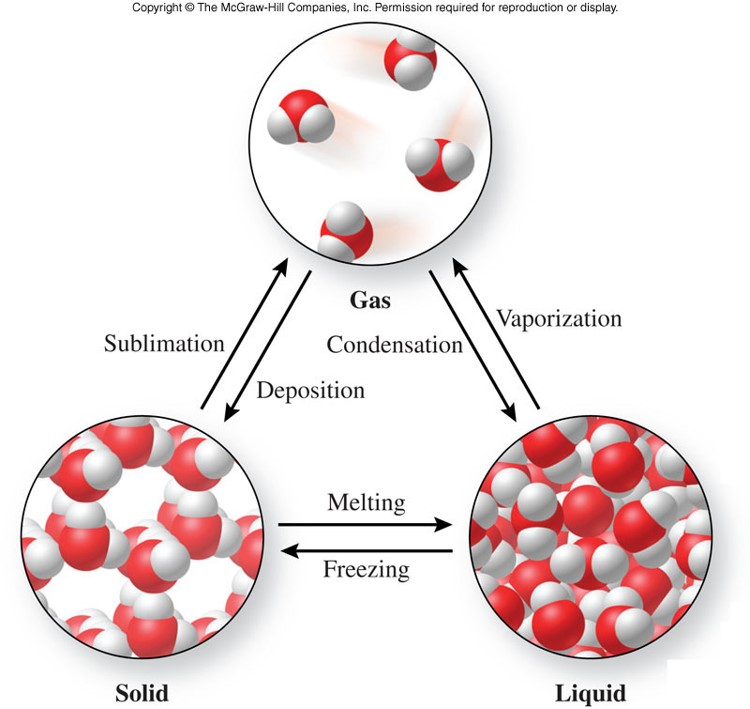
When a solid changes its state to a gas without going through the liquid phase, the process is called sublimation. But there are some processes in which solids can be directly converted to gas or gas can be converted to solid. As we generally know, that solid has to get into the liquid phase to reach the gaseous state. Whenever the substances are subjected to certain conditions, change in phases takes place. The six different changes of phases of matter which happens in between the substances are: There are six different phase changes in the states of matter.Ī phase is a distinctive form of a substance, and matter can change among the phases. These changes can take place upon adding some external energies, temperature, and pressure. We all know that matter exists in different forms in our nature. As we know, that matter exists in four states: Solid, liquid, gas, and plasma, but on earth, matter exists in only three states. All matters are made up of tiny particles, such as atoms, molecules or ions. The matter is anything that has mass and occupies space. Matter cannot always be seen.


 0 kommentar(er)
0 kommentar(er)
